Featured
The Average Kinetic Energy Of The Particles Of A Substance
The Average Kinetic Energy Of The Particles Of A Substance. Based on the kinetic molecular theory, average kinetic energy of particles. For example, the particles in a sample of hydrogen gas at 200 k have twice the average kinetic energy as the particles in a hydrogen sample at 100 k.
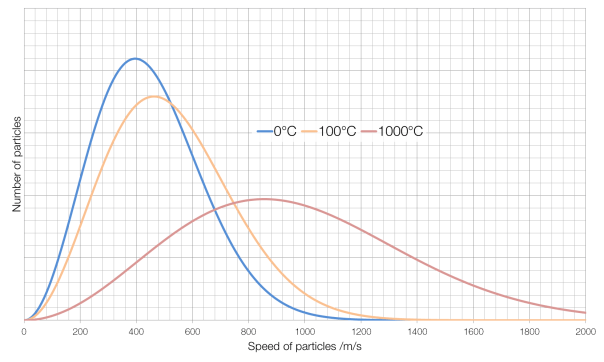
The average kinetic energy of particles in an object is also known as temperature. Which term is defined as a measure of the average kinetic energy of particles in a sample? The kelvin temperature of a substance is directly proportional to the average kinetic energy of the particles of the substance.
This Is Useful For Creating Materials Where A.
What is the average kinetic energy of a substance? The average kinetic energy of particles in an object is also known as temperature. Observe the things around you.
The Kinetic Energy, Thermal Energy, And Temperature Of A Substance Decrease When The Particles In The Substance Slow Down.
The average kinetic energy of the particles in a gas a. The average kinetic energy of gas molecules is directly proportional to absolute temperature only; If the temperature doesn’t change, the average ke doesn’t change.
Find An Answer To Your Question The Average Kinetic Energy Of A Particles In A Substance Is Called_____ Malavikamalu8242000 Malavikamalu8242000 4 Weeks Ago Science Secondary School Answered The Average Kinetic Energy Of A Particles In A Substance Is Called_____ 2 See Answers.
The kelvin temperature of a substance is directly proportional to the average kinetic energy of the particles of the substance. Helium gas liquefies at 4 k or four degrees above absolute zero. You can measure this by measuring the temperature of the object under study.
This Implies That All Molecular Motion Ceases If The Temperature Is Reduced To Absolute Zero.
As the temperature of a substance rises, the average kinetic energy of the particles making up the substance (1) increases, (2) decreases, (3) remains the same. The kelvin temperature of a substance is directly proportional to the average kinetic energy of the particles of the substance. Add your answer and earn points.
For Example, The Particles In A Sample Of Hydrogen Gas At 200 K Have Twice The Average Kinetic Energy As The Particles In A Hydrogen Sample At 100 K.
Is not affected by the temperature of the substance. If your particle is moving at the speed of ‘v’ relative to static vacuum medium then its; The kelvin temperature of a substance is directly proportional to the average kinetic energy of the particles of the substance.
Popular Posts
What Is The Average Screen Time In Australia
- Get link
- X
- Other Apps
Comments
Post a Comment